OUR LEADING PROGRAM: SEPSIS/BSI AND AMR
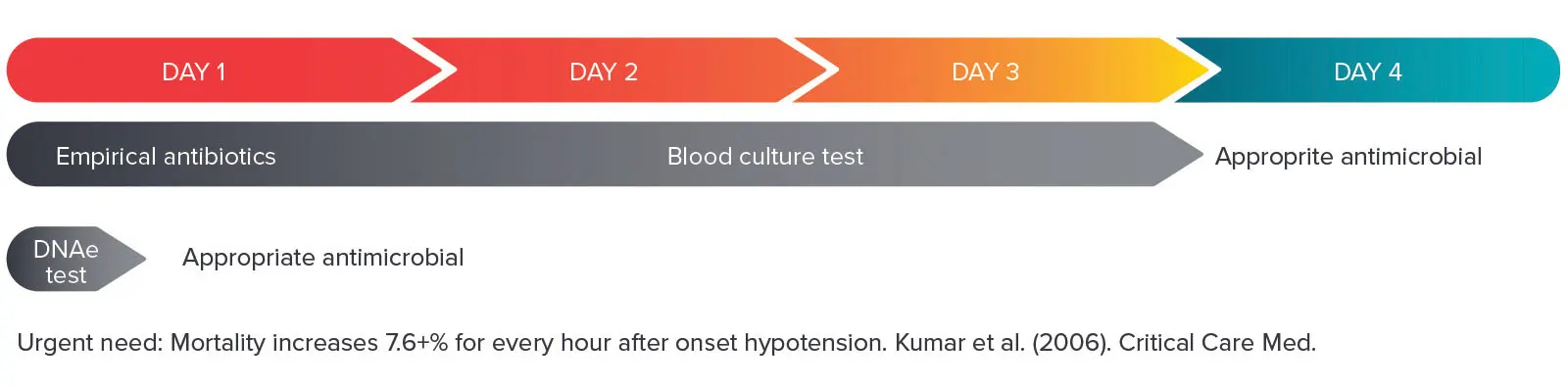
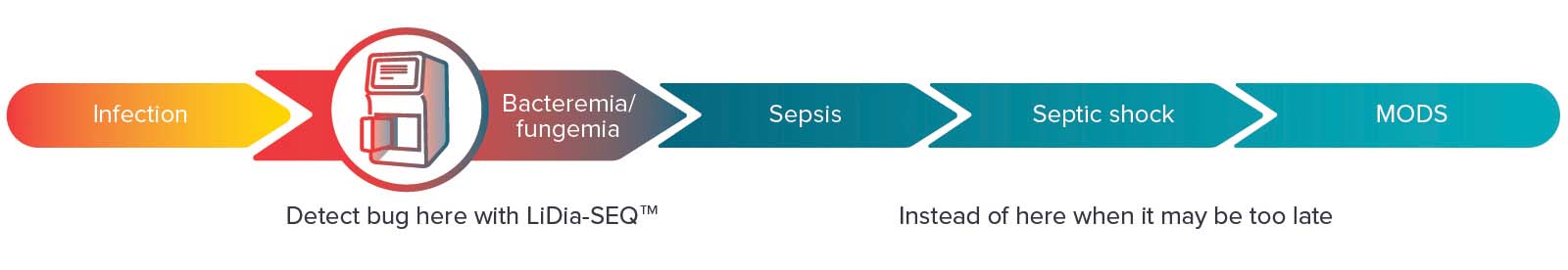
DNAe’s initial focus is on developing infectious disease diagnostics, where speed and DNA sequence information can make the difference between life and death. This includes a range of tests, starting with a groundbreaking test for bloodstream infections (BSI) and antimicrobial resistance (AMR), which uses whole blood samples to detect and identify infection causing microorganisms that can lead to sepsis.
The diagnostic will rapidly identify what infection a patient has, as well as associated AMR genes. This will provide clinicians with actionable information to help select the appropriate antimicrobial treatment.
ONCOLOGY
Also in the development pipeline, DNAe is applying its platform for cancer testing and monitoring. About 1 in 5 people develop cancer in their lifetime. The latest annual data highlight the growing global burden of cancer.
- Estimated 20 million new cancer cases every year
- 9.7 million cancer-related deaths annually
- Worldwide over 35 million new cancer cases are predicted each year by 2050, a 77% increase
*International Agency for Research on Cancer (IARC), 2024
DNAe’s integrated, sequencing-based technology will bring genomic analysis to the point-of-need, enabling testing to move out of specialist laboratories and closer to the patient. By detecting and sequencing tumor DNA directly from raw blood samples in a matter of hours, DNAe’s platform has the potential to improve cancer care, for example by detecting cancer recurrence earlier and assessing patient response to therapy.