LiDia-SEQ™ – Rapid Diagnostics at the Point-of-Need
Our system
We are the first true, point-of-need, next-generation sequencing company.
At DNAe, we are leading the way in rapid, near-patient sequencing testing that will revolutionize patient care for a range of unmet clinical needs.
We have pioneered the use of semiconductor sequencing to build a simple-to-use diagnostic platform that delivers rapid results direct-from-sample. The platform consists of a user friendly software interface, our cartridge based tests, as well as the LiDia-SEQ system which performs genomic analysis on a microchip.
DNAe was awarded a contract worth up to $51.9M by the Biomedical Advanced Research and Development Authority (BARDA)*, to develop its semiconductor sequencing diagnostic platform initially for antimicrobial resistant infections in September 2016. The development program is on track with the Company achieving pivotal key milestones in 2024, with continued support in part from the company’s ongoing contract with BARDA. The data gathered demonstrates its potential as a game-changer in the bloodstream infections (BSI) and sepsis field, as the first truly sample-to-result sequencing platform in the world, and the first solution to deliver the combination of a comprehensive clinically actionable report directly from whole blood within a work shift. The company is in the latter stages of development and preparing for commercialization.
Watch this video to hear from DNAe’s Co-Founder Professor Christofer Toumazou and CEO Sam Reed
OUR MISSION
Our mission is to make DNA sequencing rapid and accessible to transform patient care at the point-of-need, across a broad range of serious diseases.
THE CHALLENGE
INFECTIOUS DISEASE AND ANTIMICROBIAL RESISTANCE
There is a growing urgency to tackle the alarming rise of antimicrobial resistance (AMR). AMR is one of the top global public health and development threats contributing to 4.95 million deaths.1 New forecasts indicate AMR will cause 39 million deaths between 2025 and 2050 – which equates to three deaths every minute. Studies project this will rise steadily in the coming decades, increasing 70% annually by 2050.2
Testing plays a key role in reducing AMR by improving diagnosis of infections to prevent unwarranted use of antibiotics to treat BSI such as sepsis and to rapidly detect and contain resistant infections. The misuse of antibiotics can allow drug resistance to develop, therefore a timely diagnostic helps confirm the cause of an infection and guide patient management, reducing the instances of unwarranted antibiotic use. Rapid diagnosis of infections like sepsis are an urgent need as mortality increases 7.6% for every hour after onset of hypotension.3
Diagnostic solutions using fast and powerful genomic analyses
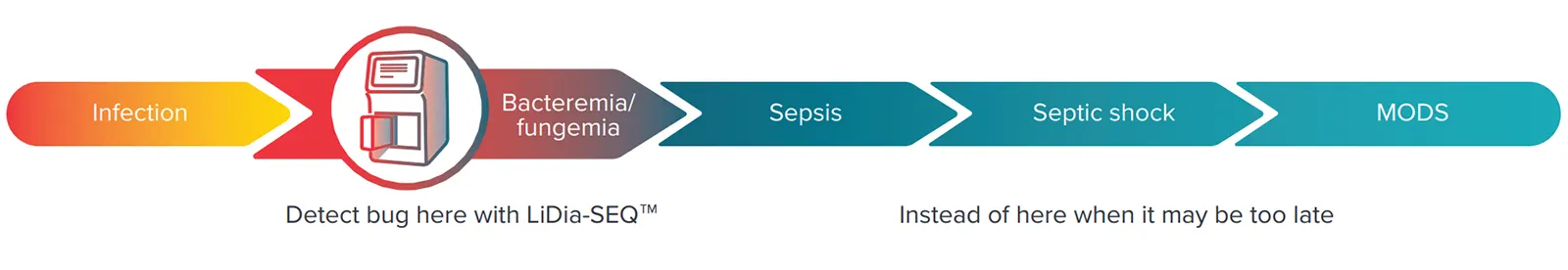
The LiDia-SEQ™ system is a direct-from-sample diagnostic platform that performs DNA sequencing on a microchip, to provide rapid, actionable information to clinicians. The user-friendly system is designed to operate in a variety of environments enabling point-of-need testing.
Our initial focus is on infectious disease diagnostics, where speed and microbial DNA sequence information can make the difference between life and death. This includes a range of tests, starting with a groundbreaking test for BSI and AMR, which uses whole blood samples to detect and identify infections that lead to sepsis. To follow this, we have a growing pipeline of tests for other healthcare applications where rapid point-of-need diagnostics are of critical need, including cancer testing and monitoring, and beyond.
Identify the infection faster

For Research Use Only. Not for use in diagnostic procedures. For illustration purposes only.
- *Acknowledgement: This project has been supported in whole or in part with federal funds from the Department of Health and Human Services; Administration for Strategic Preparedness and Response; Biomedical Advanced Research and Development Authority (BARDA), under contract number HHSO100201600017C.Disclaimer: The DNAe BSI/AMR test and LiDia-SEQ™ platform are under development and have not been approved or cleared by the FDA or any other regulatory agency.
- References:
- 1 Antimicrobial Resistance Collaborators. (2022). Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet; 399(10325): P629-655. DOI: https://doi.org/10.1016/S0140-6736(21)02724-0
- 2 Global burden of bacterial antimicrobial resistance 1990–2021: a systematic analysis with Forecasts to 2050 Naghavi, Mohsen et al. The Lancet, Volume 404, Issue 10459, 1199 – 12263.Kumar et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006;34:1589-96. 10.1097/01.CCM.0000217961.75225.E9
- 3 Kumar et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006;34:1589-96. 10.1097/01.CCM.0000217961.75225.E9
